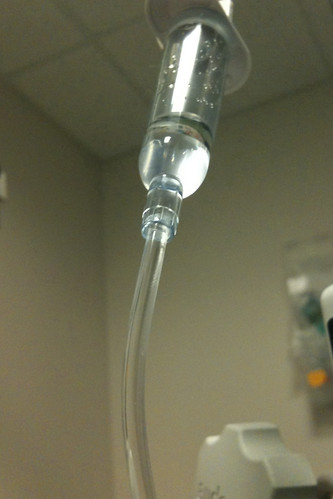
 The Symbios GOPump Rapid Recovery System is a disposable local pain management system distributed by B. Braun Medical Inc. and manufactured by Symbios Medical Products out of Indianapolis, Indiana. The GOPump System delivers pain medication slowly to a surgical site through tubes leading from a small balloon that is inflated with a local anesthetic medication.
The Symbios GOPump Rapid Recovery System is a disposable local pain management system distributed by B. Braun Medical Inc. and manufactured by Symbios Medical Products out of Indianapolis, Indiana. The GOPump System delivers pain medication slowly to a surgical site through tubes leading from a small balloon that is inflated with a local anesthetic medication.
The Food and Drug Administration (FDA) has issued a Class I recall for the Symbios GoPump in the GoPump Rapid Recovery System Kits and the GoBlock Kits, as well.
The FDA reports those systems manufactured before July 2012 and distributed from April 27, 2011 to April 30, 2013, may have excessively high flow rates. These high flow rates could result in medications being delivered too quickly from the balloon to the surgical site and could cause patient toxicity because of too much medication in too short of a time. The patient could then suffer serious illness, including seizure, abnormal heart rhythms and death. Those most susceptible are patients with a low body mass and the elderly.
When the FDA issues a Class I recall – the most serious type of recall – the agency feels there is a good chance the recalled product could cause serious injury or even death.
Of Interest:
The FDA commented that customers who purchased these devices were notified by letter dated May 10, 2013 about the problem, and then again by follow-up letters on May 14th and May 30th, as well. The FDA directed customers to quarantine the recalled product and to also complete and return a verification form.
B. Braun notified customers on May 14, 2013, and directed them to complete and return the attached “Product Removal Acknowledgement Form.” Customers who purchased the product from B. Braun Medical, should contact the company at its toll free number. Those customers who purchased this product directly from Symbios Medical Products should call the company’s toll free number for directions on returning the product and obtaining a refund.

